




Recommended Tourniquet Cuff Selection
Determine the patient’s limb shape using diagrams below. Measure the patients limb circumference, then refer to the Limb Circumference Chart for cuff selection.
To ensure the selected cuff is the correct size for the patient, verify there is sufficient overlap for the Secondary Fastener (located on underside) to fully engage with the loop material on surface of the cuff. Secondary Fasteners provide added stability to the cuff during use.
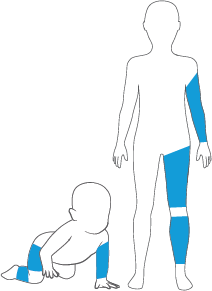
Select a Pedi-Fit or Petite-Fit Cuff and Matching Limb Protection Sleeve
For neonates, children, and adults with small limbs, select a cylindrical cuff having the most appropriate width for surgical exposure.
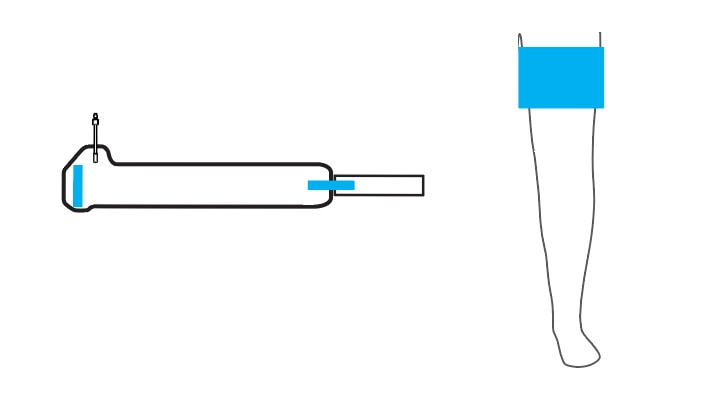
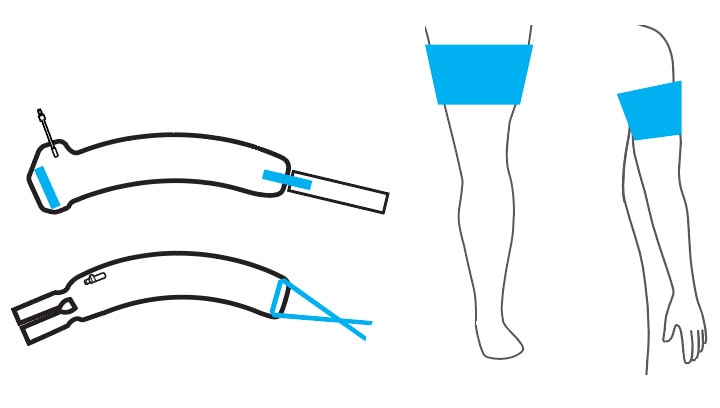
Select an Easi-Fit or Vari-Fit Contour Cuff and Matching Limb Protection Sleeve
A unique contour shape and pivoting fasteners allow the cuff to conform to a variety of tapered (conical) limb shapes.
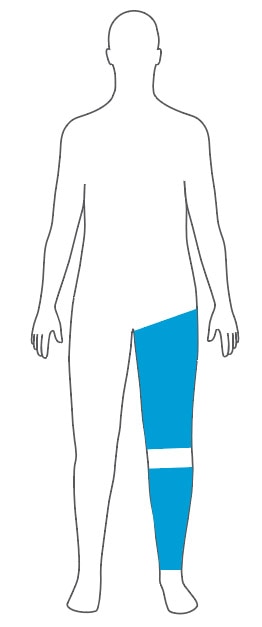
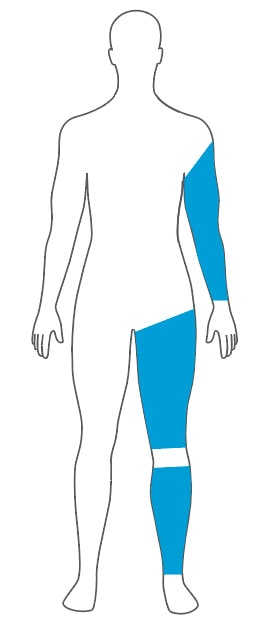
Select an Easi-Fit Cylindrical Cuff and Matching Limb Protection Sleeve
A cylindrical cuff provides the best fit for cylindrical shaped and bariatric limbs.

WARNING: For Delfi’s PTS tourniquet instruments and cuffs, always follow manufacturer’s detailed instructions.
Recommended Tourniquet Cuff Application
This Guide was prepared in consultation with James McEwen, PhD, the inventor of automatic tourniquets, to summarize relevant published guidelines and recommendations.*
Select the most appropriate tourniquet cuff considering limb size and shape, and the specific procedure:
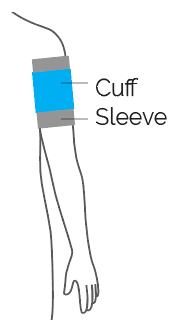
Use limb protection, ideally a sleeve specifically matched to the selected cuff.
A limb protection sleeve matched to the size and shape of the selected cuff provides the best protection against skin wrinkling, bruising and other soft tissue injuries to the limb beneath the cuff.*
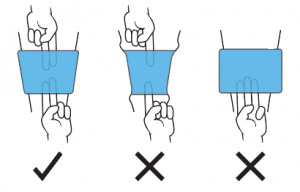
Apply the selected tourniquet cuff snugly on the limb, over limb protection.
A snugly applied cuff allows two fingers easily under the cuff at both proximal and distal edges. If only one finger fits, the cuff is too tight; if three fingers fit, the cuff is too loose.
A loose cuff will require higher pressure to stop blood flow and may slide to an unsafe position on the limb during use. A tight cuff could impede venous return of blood from the limb even when deflated.
After cuff application, the limb distal to the cuff should be exsanguinated by elastic bandage or elevation, as determined by the surgeon.
An automatic tourniquet instrument with modern safety features and audio-visual alarms should be used with the cuff.
The optimum tourniquet pressure for each patient and cuff application can be set or checked by measuring Limb Occlusion Pressure (LOP).*
Intraoperative monitoring of tourniquet safety parameters reduces the risk of complications. During the procedure, it is important to monitor tourniquet pressure and time, and to minimize both.
Upon final deflation of the tourniquet, the tourniquet cuff and underlying limb protection should be immediately removed from the limb.
* Refer to www.tourniquets.org for an expanded guide and relevant references.
WARNING: For specific tourniquet instruments and cuffs, always follow manufacturers’ instructions.
Additional Information and Links
www.aorn.org
AORN is the Association of periOperative Registered Nurses, a professional organization of whose mission is to promote quality patient care by providing its members with education, standards, services, and representation.
www.zimmerbiomet.com
Zimmer Inc. is a leading supplier of tourniquet systems, including instruments, cuffs and accessories, and Zimmer is the exclusive distributor of some Delfi products.
The following certificates and licences have been issued for Delfi and its products by national and international regulatory and standards conformance assessment bodies:
NSAI - I.S. EN ISO 13485:2016 Europe
NSAI - MDSAP ISO 13485:2016 Canada
Health Canada - Medical Device Establishment License
Health Canada - Medical Device License – Reusable Tourniquet Cuffs
Health Canada - Medical Device License – Single Use Tourniquet Cuffs
Health Canada - Medical Device License – PTS II Portable Tourniquet System
TUV - Electrical Safety Certificate – PTS II and PTS for BFR Tourniquet Systems (Canada & US)
IEC - Electrical Safety Certificate – PTS II and PTS for BFR Tourniquet Systems (International)
TUV - EMC Guidance & Declaration – PTS II Portable Tourniquet System
NSAI - CE Mark Pneumatic Surgical Sterile Single Use Tourniquet Cuffs
NSAI - CE Mark Pneumatic Surgical Sterile Disposable Contour Tourniquet Cuffs
Advanced touniquet solutions for the best in personalized care.
106-1099 West 8th Ave
Vancouver, BC V6H 1C3 Canada
1 (800) 933-3022 Toll Free in North America
+1 (604) 742-0600 Tel
+1 (604) 742-3800 Fax
2021 Delfi Medical Innovations Inc.
| site by ravensfoot